As Section of its resolve of no matter whether permitting the advertising of a completely new tobacco solution might be APPH, FDA need to find a way to ascertain the likely well being hazards of The brand new tobacco item. Although this rule isn't going to always have to have applicants to perform new research for that needs of software acceptance and filing (further than the necessities of proposed § 1114.27(b)(1)(ii)), FDA expects that PMTAs would supply adequate evidence to support the issuance of the marketing get where by they comprise info from various resources, like the two scientific and nonclinical investigations that give FDA detailed specifics of the products's probably overall health consequences inside the U.
will setting up utilizing the products completely then change to or change again to other tobacco items that may well present greater risks to particular person wellness; and
Consideration: As being the manufacturer needs the serial variety to supply a substitution, we remarkably propose you retain the first packing box or take a picture from the code just before discarding it. Thank you!
Geek Bar Wondar 1 Package is suitable for MTL lovers, the compact pen-design and style vape is paired with a chargeable 650mAh battery and forty five-minute quickly recharging capability. Linked which has a transparent pod, you could refill through a facet filling method with your favourite freebase or nic salt e-liquid.
While not necessary for software acceptance or filing less than proposed § 1114.33, FDA endorses that an software contain a dialogue of the toxicological potential with the tobacco product to trigger additional Continual toxicities, in addition to Individuals outlined earlier mentioned, such as any stop-organ toxicity or route of administration effects.
A description with the variations created for the production, amenities, or controls, if any, over the reporting period. This description might be required to incorporate enough data for FDA to find out whether or not a alter on the manufacturing, facilities, and controls results in a brand new tobacco product or could perhaps demand the promoting purchase to generally be withdrawn.
that the full excess weight of evidence—from a number of types of scientific tests, executed by investigators from various disciplines, and utilizing info from numerous international locations—demonstrates a causal marriage involving tobacco promotion and marketing and elevated tobacco use.
and insert the docket amount, located in brackets from the heading of the document, in to the “Search” box and follow the prompts and/or Visit more info the Dockets Administration Workers, 5630 Fishers Lane, Rm.
○ The wellbeing hazards from the tobacco merchandise to the two buyers and nonusers from the item and whether or not the tobacco product or service provides fewer wellbeing threat than other tobacco merchandise, like the risk of cancers ( e.g.,
carbon monoxide poisoning from waterpipe use, the ingestion of nicotine contained in e-liquids) including by means of accidental or unintended exposures, an applicant need to justify how the products could consist of these types of constituents And exactly how allowing its advertising could be APPH. This could consist of an outline of the design features, such as youngster-resistant packaging for e-liquids, that will avoid exposures to constituents that might bring about acute toxicity as Portion of proposed § 1114.
As well as the parameters that will be demanded by the proposed rule, FDA recommends a PMTA for an Finishes also include things like the following extra style parameters as described in Desk 19a and is particularly precisely requesting general public comments on no matter whether these parameters really should be necessary under the ultimate rule.
The producing area of the PMTA must comprise the following information and facts while in the producing segment to fulfill the necessities of proposed § 1114.seven(j) and that can help FDA ascertain if it conforms to the requirements of section 906(e) of your FD&C Act:
The kind of PMTA. The applicant will be required to condition the type of PMTA the applicant is distributing (
A resubmission should also comprise application sections that comprise details included by cross-reference for the PMTA for the initial tobacco product or service. It is vital to notice that these cross-referenced sections needs to be accompanied by the entire text of any updates or additional facts which can be essential to tailor this details to The brand new tobacco product.
 Dylan and Cole Sprouse Then & Now!
Dylan and Cole Sprouse Then & Now! Anthony Michael Hall Then & Now!
Anthony Michael Hall Then & Now! Brandy Then & Now!
Brandy Then & Now!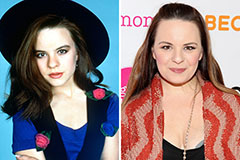 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! Nicholle Tom Then & Now!
Nicholle Tom Then & Now!